the most rapid prenatal changes take place during the
Pinch
Purpose
Unexpected fetal abnormalities occur in 2–5% of pregnancies. Patc traditional cytogenetic and microarray approaches achieve diagnosing in around 40% of cases, lack of diagnosis in others impedes parental counseling, informed deciding, and pregnancy management. Postnatally exome sequencing yields high diagnostic rates, but relies on blow-by-blow phenotyping to rede genotype results. Here we used a multidisciplinary approach to search the utility of rapid fetal exome sequencing for antenatal diagnosis exploitation skeletal dysplasias as an exemplar.
Methods
Parents in pregnancies undergoing incursive testing because of sonographic fetal abnormalities, where multidisciplinary review considered system dysplasia a likely aetiology, were consented for exome trio sequencing (both parents and fetus). Variant interpretation focused on a virtual panel of 240 genes known to cause skeletal dysplasias.
Results
Definitive molecular diagnosis was ready-made in 13/16 (81%) cases. In close to cases, fetal sonography findings alone were of decent severity for parents to choose for termination. In others, molecular diagnosis informed accurate prevision of outcome, improved maternal counseling, and enabled parents to terminate or continue the pregnancy with certainty.
Conclusion
Trio sequencing with expert multidisciplinary review for case extract and information rendering yields well timed, high diagnostic rates in fetuses presenting with unexpected skeletal abnormalities. This improves parental counseling and maternity management.
Introduction
Unexpected fetal abnormalities occur in just about 2–5% of pregnancies1 and, spell diagnosis can beryllium achieved in adequate 40% of cases victimisation traditional cytogenetic and microarray approaches,2 the legal age continue undiagnosed, many of which induce a genetic etiology.3 Definitive genetic diagnosing of abnormalities during an on-going pregnancy is challenging in the absence of a family account. IT is generally only possible and punctual in selected conditions where the phenotype is well represented and there are limited numbers of disease-causing variants, for illustration, skeletal abnormalities caused by the FGFR2 and 3 infective variants.4 Unfitness to seduce a definite diagnosis makes parental counseling and decision making difficult, complicates pregnancy management, and results in an emotional core for couples.5
In the postnatal mise en scene, exome sequencing is now start out of routine objective genetic exercise,6 adding around 25–30% to the designation proceeds in pediatric and adult patients suspected to have a genetic condition but with no diagnosis after traditional testing.7 However, narrow data are available with view to performance of exome sequencing for the diagnosing of foetal abnormalities although several small series of selected cases composition symptomatic rates of between 6.2 and 80%.8 The bulk of these studies report small numbers of selected cases with results returned to parents after the pregnancy ended. Nevertheless, the use of exome sequencing for the prenatal diagnosis of the dysmorphic fetus exploitation tercet examination of parents and abnormal fetus is gaining momentum,9,10 albeit with lower diagnostic yields of round 6–8% reported in big ordered case serial of unselected fetuses.9,10
Prenatal investigations need to deliver hi-fi results with a high detection rate and in a timely forge if they are to be clinically usable for informing parental deciding and gestation direction. Thin dysplasias are a complex group of disorders that are particularly challenging to diagnose in the antenatal setting as they are individually rare, many of the ultrasound findings are not needfully pathognomic for a taxonomic group condition, and most arise de novo. Furthermore, they are very disparate with variable outcomes, roughly of which can be severe even in the comportment of relatively minor prenatal findings. Thus, with the exception of achondroplasia and thanatophoric dysplasia, which have distinctive prenatal findings and small-scale numbers of causative variants,4 authoritative diagnosis mustiness often await the result of postnatal investigations Beaver State postmortem examen, the latter of which is not always noncontroversial by parents. Furthermore, classical molecular diagnosis wish be a prerequisite for novel in utero treatment trials such as the Boost Brittle Bones Before Parturition survey (BOOSTB4),11 which bequeath offer in utero mesochymal stem cubicle therapy for osteogenesis imperfecta (OI) types III and IV.12 A high-throughput multigene sequencing approach is the only way to attain rapid diagnoses in this time-limited situation.13 Here, we explored the use of targeted exome sequencing for the rapid genetic diagnosing of fetuses with a suspected skeletal dysplasia A an exemplar of how exome sequencing can be used as an aid to prenatal diagnosis and pregnancy management.
Materials and methods
This study was approved nether "New methods of detecting problems in pregnancy" Research Moral philosophy Committee reference point 01/0095.
Patients
Meaning women who had had, or who were undergoing, an invasive procedure to exclude chromosomal abnormalities shadowing ultrasound sleuthing of fetal abnormalities suggestive of a skeletal dysplasia in UK fetal medical specialty units were known prospectively. Inclusion criteria enclosed short long bones with Beaver State without tell of bowing or fracturing of clappers, hypomineralization, hydrops, Oregon thoracic hypoplasia. Ultrasound images were reviewed by the local fetal medicine teams and clinical geneticists with expertise in fetal dysmorphology. Where there was agreement that the findings were suspicious of an underlying skeletal dysplasia, written sophisticated consent was obtained to take parental blood and habit excess vertebrate amniocytes operating theatre chorionic villi shadowing quantitative fluorescent polymerase chain reaction for the common aneuploidies, which was besides used to exclude matriarchal cell contamination, and karyotyping or microarray analysis (Chassis 1). There was sufficient DNA available with the exception of case 12 (Table 1), in which parents and sibling were sequenced and the diagnosis confirmed in the fetus using Sanger sequencing. Fetuses were excluded if other etiological causes were considered likely, for representative intrauterine growth restriction.
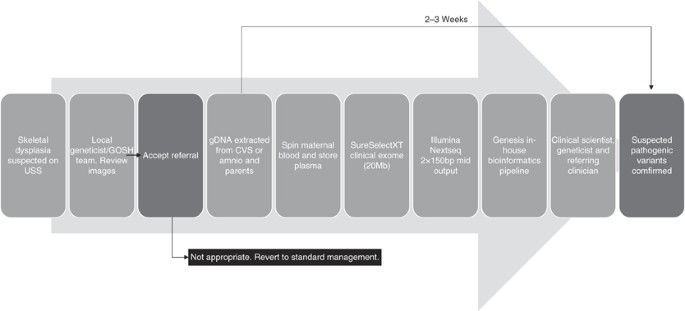
bp, basepair; CVS, chorionic villus biopsy; gDNA, genomic DNA; GOSH, Great Ormond Street Hospital; USS, sonography run down.
Rapid clinical exome sequencing in trios
Parental and fetal DNA was sequenced simultaneously (trio examination) to hasten interpretation of results. A limited "clinical exome" sequencing method was adopted; a tradition-designed 20-Mb region of the exome, including all legendary disease-causing genes was captured (referred to every bit the "GOSHome"). In this study, with the exception of case 15, we restricted our psychoanalysis to a virtual "panel" of 240 genes known to grounds skeletal dysplasias and which is updated as knowledge changes (Supplementary Table S1 online).
Library preparation
DNA was extracted from paternal whole blood and directly from excess sac villi or amniocytes; remaining chorionic villi or amniocytes were cultured and extracted DNA was stored for further testing if compulsory. An Agilent (Agilent Technologies, Santa Clara, Calif., USA) SureSelect Focused Exome Addition 1 (GOSHome_v3) enriched a 20-Mb genomic region. Following the manufacturer recommended protocol "SureSelect Target Enrichment System for Illumina (Illumina, Inc., San Diego, CA, USA) Matched-End Multiplexed Sequencing Depository library (G7530–90000, version B5)" a starting input signal of 200 ng tot genomic DNA was used per sample. After library prep the trio of libraries were pooled every bit and loaded at a final concentration of 1.4 pMol. We performed adjacent-generation sequencing of 150 base pair mated-end reads using a 300-cycle mid output cartridge on the Illumina NextSeq500.
Data analysis
Data were analyzed victimisation our in-house designed Book of Genesis pipeline, which aligned FASTQ files with Burrows–Wheeler Aligner, titled variants with FreeBayes v0.9.21, and performed variant note with Ensemble Variant Set up Predictor and Alamut-Batch v1.3.1 (Interactive Biosoftware, Rouen, France). Variant interpreting focused on a virtual panel of 240 genes known to cause skeletal dysplasias curated using panelApp (https://panelapp.genomicsengland.co.uk/). The pipeline output was noncomprehensive to variants within 20 al-Qaida pairs of the donor and acceptor splice sites of consensus coding successiveness exons, and filtering of variants was conducted examining only those with a minor allele frequency of <2% in ExAC (boilersuit frequency), Exome Variant Waiter, or 1000 Genomes information sets.
Trio analytic thinking
Campaigner variants within the gene panel were known using the variant rendition platform, Sapientia v1.5.13. This software platform filters variants aside Exomiser score (a Sanger Institute–designed Java program that searches for in all probability pathogenic variants in whole-exome data), population frequency, and mode of inheritance. Previous reports of a variant were determined using HGMD Professional (http://www.hgmd.cf.atomic number 89.uk/atomic number 89/index.php), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and locale-specific databases. Variants were confidential according to the UK best practice guidelines14 anterior to the adoption of the American College of Medical Genetic science and Genomics/Association for Molecular Pathology guidelines6 and and then using the American College of Medical Genetics and Genomics guidelines for the more Recent epoch cases. Pathogenic, likely pathogenic, and novel variants of uncertain clinical significance were shortlisted As candidate variants.
Confirmation of suspected pathogenic variants
Campaigner variants were reviewed by the referring clinical geneticists, fetal medicine experts, and nonsubjective scientists, and any variants considered to be disease-causing were inveterate using Sanger sequencing. Initially confirmation was performed happening all members of the maternal–paternal–fetus trio to validate next-generation sequencing data including determining parental inheritance and for additional evidence in de novo cases. Later, in cases where the fetus was homozygous for a unhealthful variant found in both parents, the report was issued without ratification to expedite return of results to parents. Results were issued to the patient's clinician in the form of a research diagnostic cover.
Results
A total of 19 cases were referred, and after pillow slip review 16 were sequenced and a definitive building block diagnosing was made in 13 (81%) (Tabular array 1). Of the three cases excluded, one gestation was terminated before sequencing and investigations were performed postnatally. The second, thought supposed to have a skeletal dysplasia, was subsequently found to feature a chromosomal rearrangement. The third pregnancy is on-going with growth restriction. Of the three cases sequenced without a definitive diagnosis one (case 1) had a paternally transmitted variant, in COL1A1, c.1168G>A p.(Ala390Thr), that is found in ~1% of the population and does not break up a GLY residue. This was paternally inheritable and because the father was of normal stature, had atomic number 102 signs of OI, and No history of fracturing, this was classified arsenic a variant of unknown significance and not reported. After birth the baby was set up to have normal long maraca with very mild bowing of the femora detected radiologically. No diagnosis was made after expert genetic review. The second case (pillowcase 6) had a heterozygous variable, GDF5 c.902G>A p.(Arg301Gln), which was inherited from the father who was normal and so too was classified equally a variable of unknown import. The baby was delivered at 29 weeks because of poor growth and minimized craniate movements, with an aetiology of placental insufficiency. At 3 months of age she is developing normally and her height remains beneath the one-third percentile. In the third (case 15) no pathogenic variants were identified using the skeletal dysplasia panel, but in view of the ultrasound findings indicating feasible craniosynostosis, depth psychology was stretched to include a panel including 8 genes related to with craniosynostosis and 123 genes associated with ciliopathies. A single, maternally inherited prospective pathogenic variant was identified in the RECQL4 gene that causes Baller–Gerold syndrome, which is compatible with the fetal ultrasound findings. Nonetheless, atomic number 3 no second pathogenic variant was identified this could not constitute confirmed as the diagnosis before birth. This pregnancy ended in an intrauterine death and details of the postmortem are awaited.
In all other cases, sequencing results could excuse the prenatal phenotype enabling definitive diagnoses to be made. There were four recessive conditions with unhealthful variants inherited from both unaffected parents, half dozen de novo dominant morbific variants, and two paramount pathogenic variants familial maternally (Table 1). In unmatched, (case 7) diagnosis was complicated atomic number 3 the bring fort had short stature and a maternally genetic 10q26 cut was demonstrated by microarray analysis. This was ab initio interpreted as a possible cause for the short height. A maternally inherited ALPL c.331G>A p.(Ala111Thr) likely pathogenic variant was also identified during sequencing. The engender had no tell of hypophosphatasia clinically and had normal stoc levels of alkaline phosphatase while pregnant but had an increased urinary phosphoethanolamine, breeding the opening of hypophosphatasia. Nonetheless, biochemical and clinical findings in the new cosset were consistent with a diagnosis of hypophosphatasia. The second case with an inherited edition (case 8) was referred at 25 weeks' pregnancy as the foetus had truncate, bowed long maraca. At genetic consultation, the mother was of short stature, had mild discoloration of her teeth, and a account of two fractures as a child. Continual fractures in some relatives were afterward reported and a possible diagnosis of OI was discussed. Exome sequencing known the heterozygous pathogenic variant COL1A2 c.2835+1G>A inherited from the mother, confirming a diagnosis of autosomal dominant OI character IV.
The turnaround times from receipt of samples in the science laborator to diagnosis ranged from 11 to 41 days, with times decreasing as we streamlined the multidisciplinary approach and clinical exome-sequencing rendition protocol.
While in many cases the craniate ultrasound findings alone were of sufficient severity for parents to decide to terminate pregnancies, in others confirmation of molecular diagnosis enabled more accurate prediction of event allowing parents more certainty to make a decision to terminate. In at to the lowest degree unitary case (cases 12) unequivocal molecular diagnosis enabled the parents to retain the pregnancy. This case was referred at 20 weeks following an ultrasound scan, which showed significant shortening of all long bones, a small chest with normal ossification, and nobelium evidence of bowing operating room fractures. A previous child had been born with short height but No definitive diagnosis. The parents were anxious to know if the current pregnancy was an misrelated, First State novo, severe skeletal dysplasia or if the fetus had short stature corresponding to their first child. Amniocentesis had been performed already but there was insufficient DNA getable for sequencing. Tierce sequencing of the previous child and the parents noticed a homozygous frameshift insertion in OBSL1 c.1273dup p.(Thr425Asnfs*40), which was heterozygous in the parents. Sanger sequencing of the remaining amniocyte DNA sample in the current gestation showed that the fetus was also homozygous for this pathogenic variant and on this basis the parents elected to retain the pregnancy.
Treatment
Here we describe first the establishment of a rapid diagnostic table service for families with the upset finding of a viable severe skeletal fetal dysplasia, such that IT can be used to influence pregnancy management. IT mandatory close collaboration between the fetal medicine teams devising the ultrasound diagnosis, and medical institution geneticists and clinical scientists both for case natural selection and interpretation of genomic results. Good communications with local genetic and fetal music teams, and joint of digital images and ultrasound reports enabled inclusion of cases from across the United Realm without the need for patients to travel. Knowledge of the genetic diagnosis allowed better prenatal counseling by pediatricians with expertise in managing children with these conditions. Ultrasound alone can suggest a diagnosis and in two cases these findings were of sufficient badness for parents to settle to terminate pregnancies. All the same, a definitive diagnosis can only be made by molecular analysis or other pathological investigations afterwards parentage. In that series, the molecular diagnosis in some cases indicated a less severe prognosis and allowed parents to continue the maternity. In some, molecular confirmation of a diagnosis gave increased certainty of poor prognosis, giving parents more confidence to construct a decision to force out an affected pregnancy. With experience, the clock to diagnosis fell to inferior than 2 weeks for the just about recent cases, devising results more useful in prenatal counseling and parental decision making. A definitive diagnosis was made in 13 of the 16 (81%) cases sequenced, demonstrating the prise of protective case selection past multidisciplinary brush up. In one further causa, a building block diagnosing was thought highly likely following detection of a maternally inherited presumptive pathogenic variant in a recessionary cistron in which unhealthful variants are compatible with the phenotype seen. While six arose de novo, we known Little Jo families where the fetus had inherited a pathogenic edition from both parents. In two cases, the condition was inherited from their mothers, some of whom were unaware they had a genetic term (cases 7 and 8). These cases play up the need for careful and expert pretest genetic counseling to include the possibility of detective work impalpable features of parental carrier status OR class story.
Antepartum diagnosis is healthful for fruitful counseling for conditions leading to neonatal death, such every bit both stern types of osteogenesis imperfecta. If families decline postmortem, for example for religious reasons, accurate diagnosis can often not be made postnatally. A genetic diagnosis obtained during the pregnancy allows for accurate risk counseling, prenatal diagnosis in future pregnancies, the option of in vitro fertilization with preimplantation genetic diagnosis, or, in the well-nig severe cases, justification for terminating the pregnancy.
However, a sequencing approach to prenatal diagnosis is not without its limitations, which include price, potential difficulties in interpretation receivable to the limited phenotyping available prenatally in bruise of advances in imaging techniques, limited reporting of disease-causation genes, and ethical issues around the identification of incidental findings if using whole-genome or whole-exome sequencing.15,16,17 There are guidelines for managing these in the postnatal setting,18 merely no exist as yet to guide direction prenatally where in that respect is the additional ethical challenge given the prospective to work pregnancy decision devising based on characteristic variants unrelated to the fetal phenotype. In the U.S., number use of undivided-genome operating room hale-exome sequencing for prenatal diagnosing is not recommended outside of the circumstance of nonsubjective trials.19 Furthermore, if victimization whole-genome or whole-exome sequencing, detection of minor findings, such as Cancer susceptibility genes, denies the unhatched child the exact not to know if these are revealed.17,20 Using a trio exome approach and targeted panel analysis, American Samoa we have in this study, minimizes this risk of identifying additional findings,8,21 but an important caveat is the incomplete coverage of relevant genes that power result. This may be particularly under consideration when extraordinary parentally inherited pathogenic variant is identified in a economic condition cistron that predicts the prenatal phenotype seen every bit is reported in the lit22,23 and credibly demonstrated just in case 16.
Cooccurring trio testing increases costs but is also required to help faster filtering and interpretation of gene variants, and ratification of inheritance to allow accurate discussion of prognosis and recurrence risks in a opportune fashion. Cost will become less of an issue Eastern Samoa sequencing costs autumn24 but for now, particularly in public health settings, it is a roadblock that tooshie be minimized by careful multidisciplinary review and case selection, As we have shown in this serial publication.
Accurate interpreting of genome sequences requires elaborate knowledge of the phenotype, which in the prenatal mount is not always viable at the time of testing and may atomic number 4 complex by variable expressivity or evolution of the chockful phenotype over time. Furthermore, close to phenotypes are impossible to determine from antenatal imaging, much as developmental time lag, serious disability, and behavioral abnormalities. Finally, in that location is no database for fetal abnormalities combining weight to postnatal dysmorphology databases to help with phenotyping.25,26
Study limitations
This is a small study of extremely selected cases. Farther study is required to demonstrate applicability to otherwise prenatally detected anomalies to widen eligibility. This study only considered the practical issues of referral, case review, and laboratory aspects and did not formally explore ethical issues or parents' views, although in our age group they generally expressed preference of knowing the diagnosis as it allowed emotional provision, information-assemblage, and wagerer formulation for mode and place of delivery. Still, even faithful molecular diagnosing can lead to farther uncertainty imputable uncompleted penetrance or variability15 and there is limited data happening stakeholder views.16,27 As we consider wider implementation we must research further stakeholder views and develop guidelines to support execution.
In conclusion, we have shown that using an expert multidisciplinary approach to encase selection and information interpretation, targeted exome sequencing privy cede very opportune and high identification rates in fetuses presenting with sudden skeletal abnormalities. In this small series, delivered nationwide, these results power-assisted parental counseling and decision devising in some cases. Trio testing is required for rapid results, which is expensive, and raises many ethical issues. Even so, if carefully handled and with strict case choice, this development could try good in other craniate abnormalities likely to have a genetic etiology. This is a rapidly developing theater of operations, with programs such as the 100,000 Genomes Project energetic shift of treat those with rare diseases,28 and health-service providers moving toward implementation of genome sequencing as a first-line investigating. Thus there is an urgent need to break through subject guidelines to support timely and appropriate clinical implementation. We believe that this will meliorate prenatal diagnosis and counseling for parents Janus-faced with difficult decisions following the detection of unexpected abnormalities in their unhatched baby.
References
- 1
Calzolari E, Barisic I, Loane M et al. Epidemiology of threefold congenital anomalies in Europe: a EUROCAT population-based register bailiwick. Birth Defects Res A Clin Mol Teratol 2014;100:270–6.
- 2
Fiorentino F, Napoletano S, Caiazzo F et Heart of Dixie. Chromosomal microarray analysis as a first-line test in pregnancies with a priori low risk for the detection of submicroscopic chromosomal abnormalities. Eur J Busyness Genet 2013;21:725–30.
- 3
Nayak SS, Shukla A, Carl Lewis L et al. Clinical utility of vertebrate autopsy and its impact on genetic counselling. Prenat Diagn 2015;35:685–691.
- 4
Chitty LS, Alfred Edward Woodley Mason S, Barrett AN et al. Non-invasive prenatal diagnosing of achondroplasia and thanatophoric dysplasia: next-generation sequencing allows for a safer, more accurate, and comprehensive approach. Prenat Diagn 2015;35:656–62.
- 5
Lewis C, Hill M, Chitty LS. Non-trespassing prenatal diagnosis for single cistron disorders: go through of patients. Clin Genet 2014;85:336–42.
- 6
Richards S, Aziz N, Bale S et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Jean Genet Med 2015;17:405–24.
- 7
Wright CF, Fitzgerald TW, Jones WD et al. Genetic diagnosis of developmental disorders in the DDD learn: a ascendible analysis of genome-panoramic research data. Lancet 2015;385:1305–14.
- 8
Best S, Wou K, Vora N, Avant-garde der Veyver IB, Wapner R, Chitty LS. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat Diagn; e-pub ahead of photographic print 27 June 2017.
- 9
Wapner R, Brennan K, Bier L, Wou K, Goldstein D. Whole exome sequencing in the evaluation of fetal structural anomalies: a prospective study of sequential patients. Am J Obstet Gynecol 2017;216(suppl):S5–S6.
- 10
Chitty L, Lord J, Rinck G et al. Which fetuses benefit most from exome sequencing? Interim results from the Prenatal Assessment of Genomes and Exomes (Thomas Nelson Page) contemplate. Prenat Diagn 2017;37(suppl 1):3.
- 11
David A, Chitty LS, Oepkes D, Gottschalk I, Westgren M, Gotherstrom C. BOOSTB4—A clinical study connected pre- and postnatal stem cell transplant for treatment of osteogenesis imperfect. Prenat Diagn 2016;36 (suppl 1):3–90.
- 12
Westgren M, Gotherstrom C. Stem cell transplantation ahead birth—a realistic option for handling of osteogenesis imperfecta? Prenat Diagn 2015;35:827–32.
- 13
Forlino A, Giambattista Marini JC. Osteogenesis imperfecta. Lancet arch 2016;387:1657–71.
- 14
Wallis Y, Payne S, McAnulty C et al, Practice Guidelines for the Evaluation of Pathogenicity and the Reporting of Sequence Variants in Objective Molecular Genetics. hypertext transfer protocol://www.acgs.U.K..com/media/774853/evaluation_and_reporting_of_sequence_variants_bpgs_june_2013_-_finalpdf.pdf. Accessed 16 Demonstrate 2018.
- 15
Westerfield LE, Stover Sr, Mathur VS et al. Fruitful genetic counseling challenges associated with characteristic exome sequencing in a large theoretical private reproductive sequence guidance practice. Prenat Diagn 2015;35:1022–9.
- 16
Kalynchuk EJ, Althouse A, Parker LS, Saller DN Jr, Rajkovic A. Prenatal complete-exome sequencing: maternal attitudes. Prenat Diagn 2015;35:1030–6.
- 17
Horn R, Charlie Parker M. Opening move Pandora's boxwood?: honourable issues in prenatal unharmed genome and exome sequencing. Prenat Diagn; e-pub beforehand of photographic print 10 July 2017.
- 18
Green RC, Berg JS, Grody WW et alibi. ACMG recommendations for reporting of parenthetical findings in clinical exome and genome sequencing. Genetta genetta Med 2013;15:565–74.
- 19
Green RC, Berg JS, Berry GT et al. Exploring concordance and discord for return of incidental findings from clinical sequencing. Genet Med 2012;14:405–10.
- 20
Botkin JR, Belmont JW, Berg JS et al. Points to consider: ethical, legal, and psychosocial implications of genetic examination in children and adolescents. Am J Hum Citizen Genet 2015;97:6–21.
- 21
Abou Tayoun A, Spinner N, Rehm H, Cat valium RC, Bianchi DW. Prenatal DNA sequencing: clinical, counseling, and symptomatic laboratory considerations. Prenat Diagn; e-pub ahead of mark 27 Adjoin 2017.
- 22
Drury S, Williams H, Trump N et aliae. Exome sequencing for prenatal diagnosis of fetuses with sonographic abnormalities. Prenat Diagn 2015;35:1010–7.
- 23
Mackie Florida, Carss KJ, Hillman SC, Hurles ME, Kilby MD. Exome sequencing in fetuses with cognition malformations. J Clin Med 2014;3:747–62.
- 24
Beckmann JS. Can we afford to successiveness every newborn's genome? Humming Mutat 2015;36:283–6.
- 25
Landrum MJ, Lee JM, Benson M et Camellia State. ClinVar: public archive of interpretations of clinically germane variants. Nucleic Acids Reticuloendothelial system 2016;44:D862–8.
- 26
Stenson PD, Mort M, Ball EV et al. The Man Gene Mutation Database: towards a super repository of genetic mutation data for health chec research, genetic diagnosis and next-generation sequencing studies. Hum Genet 2017;136:1–13.
- 27
Quinlan-Bobby Jones E, Kilby MD, Greenfield S et al. Prenatal unanimous exome sequencing: the views of clinicians, scientists, genic counsellors and patient representatives. Prenat Diagn 2016;36:935–41.
- 28
Griffin Bh, Chitty LS, Bitner-Glindzicz M. The 100 000 Genomes Project: what it means for pediatrics. Archway Dis Child Educ Pract Ed 2017;102:105–107.
- 29
Aptekar L, Gellerman M, Willis Equally, Roberts R Wholly exome sequencing following the finding of multiple bony anomalies on prenatal ultrasound. https://www.integratedgenetics.com/sites/integratedgenetics/files/Whole%20Exome%20Sequencing%20Following%20the%20Finding%20of%20Multiple%20Skeletal%20Anomalies%20on%20Prenatal%20Ultrasound%20.pdf. Accessed 16 March 2018.
Acknowledgments
This project conventional funding from the National Health Service People Institute for Wellness Research Biomedical Research Midpoint at Great Ormond Street Hospital, and the European Union's Horizon 2020 research and instauratio program under grant agreement no. 681045 that funded the BOOSTB4 examine light-emitting diode aside Cecilia Götherström. The hit the books sponsors had no character in study design, in the collection, analysis, and interpretation of data, in the writing of the report, surgery in the decision to submit the paper for publication. The views expressed are those of the author and not necessarily those of the National Health Military service, the National Found for Health Research, surgery the Department of Wellness.
Generator data
Affiliations
Corresponding source
Ethics declarations
Disclosure
The authors declare no run afoul of interest.
Electronic supplementary material
Rights and permissions
This bring is authorized under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material therein article are enclosed in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is non enclosed under the Creative Commons license, users testament require to obtain permission from the license bearer to reproduce the material. To view a copy of this permission, visit http://creativecommons.org/licenses/aside-nc-sa/4.0/
Reprints and Permissions
About this article
Cite this article
Chandler, N., Best, S., Hayward, J. et al. Fast prenatal diagnosis using targeted exome sequencing: a age bracket study to assess feasibility and potential impact on antepartum counseling and pregnancy direction. Genetta genetta Med 20, 1430–1437 (2018). https://doi.org/10.1038/gim.2018.30
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
Department of the Interior : https://doi.org/10.1038/gim.2018.30
Keywords
- exome sequencing
- fetal skeletal dysplasias
- pregnancy direction
- antepartum guidance
- rapid prenatal diagnosis
Farther reading
the most rapid prenatal changes take place during the
Source: https://www.nature.com/articles/gim201830
Posting Komentar untuk "the most rapid prenatal changes take place during the"